Cord Blood and Medical Misinformation: The Big Business of Unproven Stem Cell Treatments
Overview
David Greene, who lost his medical license in 2009 after botched surgeries resulted in several deaths, is a marketing CEO in the United States whose lucrative business sells unproven—and sometimes dangerous—medical treatments using birth tissues.1 While stem cell therapies are effective treatments for a limited list of diseases, Greene and his marketing strategies have persuaded customers that stem cell therapy is a near cure-all. His company claims to have treated 10,000 people.2
Offline, Greene gets new clients by hosting events in hotels and conference centers. Online, his marketing relies on testimonials, recontextualized media, purchased awards, and paid advertising.
Investigation into these marketing strategies shows that Greene is profiting off a model that is based on phony science. Greene has manipulated search engines via to bury bad press and invested heavily in his own scam. In reputation management, he employs pay-for-play awards, marketing, and native so that articles about his product are integrated into otherwise reputable news coverage.3
- 1“Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes,” FDA, July 22, 2020, archived on Perma.cc, https://perma.cc/X9FP-5MUJ.
- 2Caroline Chen, “The Birth-Tissue Profiteers,” ProPublica, May 7, 2019, archived on Perma.cc, https://perma.cc/M5HL-EGBQ.
- 3R3 Stem Cell, “R3 International Now Offering Stem Cell Therapy for Coronavirus in Mexico,” AP NEWS, June 30, 2020. archived on Perma.cc, https://perma.cc/BY7K-8DUZ.
Background
Stem cells have become in the past four decades a promising potential medical treatment for certain health problems, though their application remains limited. Scientists have identified various types of stem cells. They function in different ways, although these variations are often conflated in the popular understanding of their role in medical treatments. For the purposes of this case study, it is important to clarify the major distinctions and applications of two types of stem cells: embryonic and perinatal stem cells.
Embryonic stem cells, which come from human embryos, are the most flexible. They have not yet been “fated,” as scientists put it, to become a specific cell. In their embryonic state, they have the ability to mature into any type of cell in the body. This is called “pluripotency.” Embryonic stem cells offer promising medical applications for heart disease and diabetes, and research into them continues.1
Perinatal stem cells come from birth tissues such as the placenta, amniotic fluid, the amniotic membrane, and the umbilical cord.2 Of these there are two main subtypes: Cells from the umbilical cord, called “cord blood stem cells,” which are described as “hematopoietic” because they have the potential to become blood cells; and amniotic stem cells, which come from other birth tissues and are less well-understood. Amniotic stem cells are believed to have the potential to become bone, fat, and cartilage cells.3
The different types of stem cells have specific applications in science and medicine. Embryonic, amniotic, and cord-blood stem cells are not interchangeable in existing treatments, and using one stem cell type where another is called for would be at best useless and at worst dangerous.
This case examines the marketing of perinatal stem cells as a kind of "miracle" medical treatment.
To acquire perinatal stem cells, birth tissues like the placenta, amniotic membrane, fluid, and umbilical cords are processed and stored after births for future use. Because cord-blood transplants have been used for decades, many hospitals offer programs for people giving birth to donate umbilical cords to public banks, which store cord blood for potential blood transplants. Those banks are “the only hematopoietic stem cell source to be regulated by the U.S. Food and Drug Administration [(FDA)].”4
There are also private banking options accessible only to the donor’s family. Private cord banking is marketed to pregnant people as a potentially life-saving future treatment for their children. Some of these banks hold both cord blood stem cells and amniotic stem cells.
Cord blood stem-cell transplants are of limited use, and rare. For a family to use their privately stored cord blood, they first need to know whether there is a sufficient amount of it and whether the ailment is treatable by a cord stem cell transplant.5 There are about 70 diseases, including leukemia, lymphoma, and sickle-cell disease, that are treatable.6 The use of amniotic stem cells is even rarer, and there is no known approved treatment that uses them.
Private banks process and freeze birth tissues without testing, and store it regardless of viability. If the umbilical cord blood does not have enough stem cells (a problem affecting about 75% of donated cord blood), it is used for research, discarded, or sold.7 If a person giving birth has opted not to donate or store their birth tissues, hospitals that are not affiliated with any public cord banks can choose to sell those tissues.8
Sales of umbilical cord blood led to a dangerous industry: umbilical cord tissue therapy. These therapies are not FDA approved.
David Greene and his stem cell therapy empire, which uses perinatal stem cells in unapproved treatments, is one example of this problem. Greene tells his clients that the stem cells his clinics use can morph into whatever the body needs, creating a wide yet scientifically unproven market for the product he offers.9
The nuance in types of stem cells (umbilical cord, amniotic, embryonic, marrow, etc.)10
and the still-developing research around their potential therapeutic value makes the topic confusing for people outside science and health care.
- 1 Jacquelyn Cafasso, “Stem Cell Research: Uses, Types & Examples,” Healthline, July 8, 2017, archived on Perma.cc, https://perma.cc/642L-ZJCR; Research note: Induced pluripotent stem cells (iPSCs) are the most common in medical research, and perhaps the type of stem cells with the most promising therapeutic potential. iPSCs are most commonly from blood or skin cells, but can be taken from almost any type of body cell. They are then “reprogrammed” into an embryonic-like pluripotent state. As such, iPSCs can be generated from any at any given time, and used for therapeutic purposes (Thanks to Dr. Ralda Nehme for the explanation).
- 2 Mayo Clinic Staff, “Stem cells: What they are and what they do,” Mayo Clinic, June 8, 2018, archived on Perma.cc, https://perma.cc/AZD9-GHPR.
- 3Stavros P. Loukogeorgakis and Paolo De Coppi, “Concise Review: Amniotic Fluid Stem Cells: The Known, the Unknown, and Potential Regenerative Medicine Applications,” Stem Cells, July 2017, Vol. 35, No. 7, pages 1663-1673, https://perma.cc/XK89-LD3A.
- 4Joanne Kurtzberg, “A History of Cord Blood Banking and Transplantation,” Stem Cells Translational Medicine, May 2017, Vol. 6, No. 5, pages 1309–11, https://perma.cc/5TAY-P529.
- 5Adults can be treated via cord blood stem cell transplants with two cords instead of one (per Kurtzberg, “A History of Cord Blood Banking and Transplantation”).
- 6Belle, “The Cord Blood Banking Controversy,” Parents, April 12, 2006, archived on Perma.cc, https://perma.cc/P84B-UX42.
- 7Belle, “The Cord Blood Banking Controversy.”
- 8 Ross Hauser and Danielle R. Steilen-Matias, “Misinformation surrounding amniotic, cord blood, placenta stem cell therapy,” Caring Medical Florida, accessed on November 15, 2021, archived on Perma.cc, https://perma.cc/C5PG-PJNK.
- 9Erin Allday, “What Are Stem Cells and How Do They Work?,” San Francisco Chronicle, 2018, archived on Perma.cc, https://perma.cc/BZ2F-NX42.
- 10 Antonio Romito and Gilda Cobellis, “Pluripotent Stem Cells: Current Understanding and Future Directions,” Stem Cells International, December 20, 2015, archived on Perma.cc, https://perma.cc/7RZW-XJ5T.
STAGE 1: Manipulation Campaign Planning and Origins
David Greene, who lost his medical license as a result of botched surgeries that led to several deaths, is among the most well-known peddlers about stem cells. After losing his license in 2009,1 he became an entrepreneur: Greene started Lead, a health marketing company, in 2009, and then R3 Stem Cell, a regenerative medicine clinic in 2013.2 He sells stem cell therapies using perinatal stem cells through his network of treatment centers, R3 Stem Cell Clinics.
Greene’s R3 website suggests that stem cell therapy is an easy fix for a long list of ailments, from arthritis to erectile dysfunction. Importantly, though perinatal stem cells (specifically cord-blood stem cells) are approved as treatment for some diseases, Greene’s claims amount to medical misinformation as there is a lack of evidence to suggest they can cure the illnesses he suggests they can.3 Greene uses testimonials, paid news promotions, awards, and recontextualized media as a means to pitch his product to potential patients.
His online practices complement his offline . At conference centers and hotel lobbies, he presents anecdotes about patients he has helped through stem cell therapies. These meetings with Greene are an opportunity for him to gain clients’ trust by meeting them, answering questions, and sharing “success stories”—the closest thing he has to evidence that his remedies work.
At one in-person conference, Greene told potential clients (and a ProPublica reporter), “I think of a stem cell as a blank slate, a master cell that has not made the decision of what it wants to specialize in.” The cells he advertises and injects “can turn into anything we clinically need them to become,” he promises.4
According to reporting and his own , Greene uses both amniotic and cord blood stem cells—neither of which is pluripotent, like an embryonic stem cell, and capable of becoming any kind of cell. Greene has promoted his scam via his patients, whose word-of-mouth advertising benefits him.
He has used cloaked science to increase revenue and his client base. A 2017 paper from a study in Chile describes a trial in which 15 heart failure patients got cord blood cells.5 While it did improve blood pumped per heartbeat for those 15 patients, the sample size is small and researchers have been unable to reproduce the results. Nevertheless, Greene cites this study as among the “success stories” of umbilical cord blood cells—regardless of its blood/heart-specific context.
STAGE 2: Seeding Campaign Across Social Platforms and Web
Greene has worked to seed his marketing message across the web—or to make it appear as if it has been seeded across the web. To do so, he posts testimonials, awards, and on his website and has spread paid news promotions across websites unassociated with his clinic. Through keyword squatting, he has ensured that positive press about his company shows up first.
He advertises stem cell therapies, also called “regenerative medicine,” as a surgery-free way to treat “arthritis, tendinitis, psoriasis, lupus, hair loss, facial wrinkles, scarring, erectile dysfunction, heart failure, cardiomyopathy, chronic obstructive pulmonary disease, asthma, emphysema, stroke, Alzheimer’s disease, multiple sclerosis, ALS, neuropathy, pelvic pain, diabetes, dry eye, macular degeneration, kidney failure”—and more.1
For the maladies he lists, no clinical trials or scientific studies have found that perinatal stem cells are curative. Greene acknowledges that the evidence is anecdotal, based on “a lot of success stories” instead of rigorous clinical trials and data.2 A bar running across the bottom of his webpage emphasizes that R3 does not make “ANY claims.”
Figure 1: This image is a screenshot of the banner across the bottom of R3 Stem Cell’s website. It reads: “Disclaimer and Cookie Notice: R3 is not promising a particular outcome or making ANY claims. The FDA considers stem cell therapy experimental. Results vary. We use cookies to ensure that we give you the best experience on our website. If you continue to use this site we will assume that you are happy with it,” archived on Perma.cc, perma.cc/9FGT-K5P5. Credit: TaSC.
Much like the “success stories” that Greene tells at conferences, he relies on testimonials for his website. Since there is no scientific evidence showing the efficacy of these treatments, his library of testimonials offers anecdotal evidence of umbilical cord blood stem cell treatments. Instead, he uses text and film testimonials so that potential, future patients can hear about their experiences of past patients.
These testimonials are highly curated. They uncritically recommend R3 (at its most critical, for example, one text testimonial warns, “It is not an immediate fix!! It does take a few months.. but it is remarkable to get rid of the pain!”).1 The one guardrail on the testimonials is a quick asterisk, which warns “Outcomes will vary between individuals. No claims are being made with regenerative therapies. The FDA considers stem cell therapy experimental.”2
Written testimonials share personal experiences, covering everything from claims that the procedures reverse aging (one customer from DC wrote, “I literally feel like I went back in time by 20 - 30 years”), work for years, and are “Worth every dime !”3
- 1 “Success Stories,” R3 Stem Cell, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/7FLX-DRKW.
- 2“Homepage 2018,” R3 Stem Cell, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/9FGT-K5P5.
- 3“Success Stories.”
Greene’s video collection (available on his website and on YouTube) features testimonials from Journey’s lead singer Arnel Pineda, Super Bowl Champion and former Chicago Bear Otis Wilson, and Magic Mike Live Performer Jackson, along with testimonials from Vietnam veterans, MMA fighters, and an Air Force medic. These voices amplify R3’s marketing attempts while also implying a level of luxury, since musicians and athletes are choosing R3. To balance the celebrity endorsements, R3’s website and page feature ordinary patients who have been able to resume their favorite pastimes pain free. Pineda tweets about R3—as do bot-like accounts on with numerical handles, low follower counts, and few likes.
Greene also lists his awards on his webpage. The awards that David Greene and R3 have been awarded are suspect—and likely examples of what the Better Business Bureau calls “Vanity Award Scams.”1
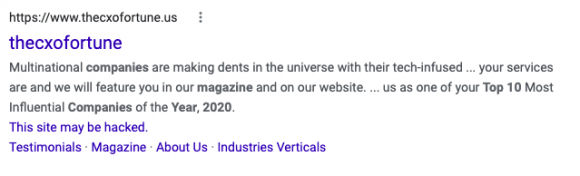
He received both “Most Innovative Company in 2020” and “Best Company in 2020” from a group called The CXO Fortune—a company registered in Wyoming in 2020 that as of September 2021 had four Twitter followers, two Facebook followers, two followers, eight YouTube subscribers, and 88 LinkedIn connections. When searched via while researching this case, a warning popped up, saying, “This site may be hacked.”2 By the time of publication, the warning had been removed. In that and other ways, the CXO Fortune’s online presence suggests that its awards are not influential ones.
- 1“BBB Tip: Vanity Awards,” Better Business Bureau, November 20, 2019, archived on Perma.cc, https://perma.cc/9PGR-BFDY.
- 2“The Cxo Fortune - Google Search,” accessed September 24, 2021, archived on Perma.cc, https://perma.cc/8J8W-67SM.
R3 was also named one of the "50 Smartest Companies of the Year" by The Silicon Review, an online business magazine. The Silicon Review’s model is pay-to-play. In 2019, it cost $2,500 to be on their list of “smart companies.”1 For this price, The Silicon Review promises that the paying company will reach an audience of 70,000 Silicon Review subscribers.2
The R3 website has an “As Featured On” section with icons from ABC, NBC, CBS, and CBS. None of these articles show up in searches for them, according to TaSC’s research, but the image on his website makes it appear as if his business has been reported on and praised across the media.
- 1Jay Valentine, “Sales SCAM Alert: Silicon Review,” Jay Valentine, April 5, 2020, archived on Perma.cc, https://perma.cc/Z45H-BDX8.
- 2Valentine, “Sales SCAM Alert: Silicon Review.”
Greene has seeded his medical misinformation campaign online by paying for deceitful advertising on local media designed to look like real news. These local news stations all subscribe to wire services, and these “advertorials” are part of the package. By purchasing press releases via PR Web and PRNewswire, this strategy buries any information about Greene’s medical certification revocation, promotes his work, and fills out an internet search so that searching his name or his business yields positive results. The “news” often comes from US Lead Network, which is his marketing company, or from R3 Stem Cell itself.
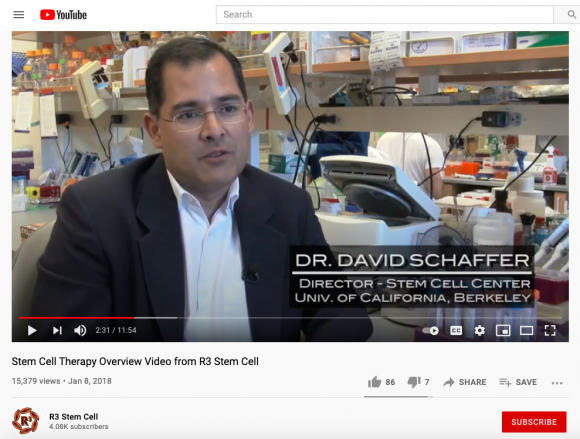
Similar to his attempts to increase legitimacy by naming his celebrity clients, Greene affiliates his product with academics using recontextualized media. On the R3 website Dr. Gary Steinberg, the chair of neurosurgery at Stanford University, and David Schaffer, the director of the Stem Cell Center at the University of California, Berkeley, are both featured—without their consent and out of context—on Greene’s website, as co-reported on by ProPublica1 and The New Yorker.2
Steinberg has been studying stem cells for over twenty years and has conducted trials to determine whether they could be used for stroke patients. Clips of his research were used by Greene to make it seem like R3’s product has been studied and tested for decades (the reality was that Steinberg’s cells came from bone marrow and were grown in the lab).
“I have no control over how these clips get used, unfortunately,” Steinberg told ProPublica and The New Yorker.3 “I’m not happy about it.” He added, “I’m an advocate for thoughtful, controlled trials, and they will never know, unless we do controlled trials, if it works.”4
The edited clip of Schaffer on the R3 YouTube page makes it appear that he is explaining regenerative medicine to and for Greene’s clients, when the reality was that Schaffer had never heard of Greene or his business and does not work with amniotic tissue.
“That footage was not included with my consent,” he told ProPublica and The New Yorker after being shown the clip of himself speaking on R3’s website. “I would be suspicious of the business.”5
Greene’s most viewed video on YouTube has 225K views. These videos are also posted to his Facebook page, which has 13.5K followers. The page is run internationally, with four managers from Pakistan, three from the United States, two from India, and one manager each from India, Macedonia, Nicaragua, and the Philippines. Since July 2019, he has run about 110 ads across his Facebook page, Instagram, and Messenger, according to Facebook’s Ad Library.1
Greene is aware of and benefits from popular misconceptions around what stem cells are and what stem cell-based treatments can achieve. In a May 7, 2019 interview with ProPublica, he “acknowledged that most patients don’t understand the difference between embryonic and other types of stem cells,” and that his language is aimed at avoiding medical terminology: “when you look at what people are typing on the web, ‘stem cell’ is the No. 1 key phrase. That is the key word that the public in America understands.”2
By conflating all stem cells in commercializing his product, Greene benefits from the very real optimism around what some stem cell treatments can offer to medicine, promising solutions that are unproven for the list of ailments he advertises.
- 1“Ad Library,” Facebook, September 23, 2021, archived on Perma.cc, https://perma.cc/83AH-AXZ4.
- 2Chen, “The Birth-Tissue Profiteers.”
STAGE 3: Responses by Industry, Activists, Politicians, and Journalists
Greene's clinics themselves do not appear to have received much authentic direct press, but he benefits from the generally positive press reported about the future promise of stem cell therapies.
This notion that stem cell therapies could be a panacea has allowed Greene to create a bigger space for himself within the industry. Greene has about 30 R3 clinics in the US, Mexico and Pakistan. Within the US, providers (offering R3 products in non-R3 clinics) in at least 186 cities have become affiliated with him since he opened his business in 2013. According to a brochure seen by ProPublica and The New Yorker, “clinics pay Greene at least $495 to join his network, another $495 per month and $75 for each prospective patient that comes to them through his website.”1 These numbers are for amniotic stem cell treatments, but they suggest that Greene gets paid with every procedure that happens within the network.
The number of clinics and providers are an indicator of how successful his scam has been.
- 1Chen.
STAGE 4: efforts
There have been several attempts to contain Greene’s messaging and mitigate against potential harms from his unproven treatments. These have largely been centered around inconsistent platform actions, critical press, and investigations.
Twitter suspended R3’s account (@r3stemcell), but his other social media accounts remain active at the time of writing. Google has banned advertisements about stem cells1 (note: Greene claims this worked to his credit, as other stem cell companies are looking to him for insight on FB stem cell advertisements).2
The American College of Gynecologists and Obstetricians,3 the FDA,4 state cord banks,5 government health services6 have all debunked the kinds of promises Greene makes and warned about the danger of unregulated stem cell therapies. Be The Match, a national non-profit organization, has online educational campaigns about the uses of cord stem cells and encourages public banking of umbilical cord blood through its website and social media.7
Claims regarding the efficacy of umbilical cord stem cell therapies—along with Greene’s other birth tissue offerings—have been mitigated by . In a multi-part investigation conducted by ABC’s Denver 7 in 2018, Keith Wonnacott, Ph.D., Chief of the Cellular Therapies Branch in FDA’s Office of Cellular, Tissue, and Gene Therapies, told the newsroom:
Because cord blood contains stem cells, there have been stem cell fraud cases related to cord blood. Consumers may think that stem cells can cure any disease, but science doesn’t show this to be the case. Patients should be skeptical if cord blood is being promoted for uses other than blood stem cell regeneration.8
ProPublica and The New Yorker collaborated on a 2019 article that used Greene’s story to talk about the network of businesses involved and people affected by his deception. In it Jeanne Loring, the Center for Regenerative Medicine at the Scripps Research Institute director and the Aspen Neuroscience chief scientific officer explained that Greene’s assertions—that any stem cell can become whatever the body needs—are not true: “If a stem cell from one organ is put into another, like a placenta or umbilical cord cell into a knee, it will die. It can’t become something else.”9
The article describes a range of outcomes that perinatal stem cell patients (not just Greene’s own) have had, ranging from no improvement to sepsis. It also reported on the conditions of a tested sample of stem cells from his vendor: there were 600,000—far fewer than the millions advertised—and less than half were alive.10
Pew has also documented the number of clinics offering unregulated and unproven services—more than 700 as of 2020—in calling for FDA regulation.11
The 2019 collaboration between The New Yorker and ProPublica was followed by an May 28, 2019 date “Untitled Letter” from the FDA to Greene (an “Untitled Letter” is a lower admonishment than a warning letter and a chance for Greene to self-correct).12 It read: “We note that your products are intended to treat a variety of serious or life-threatening diseases or conditions. Such unapproved uses raise potential significant safety concerns. Additionally, because the products are administered by various higher risk routes of administration, including IV, their use, if contaminated could cause a range of adverse events.”13
Two days later, the FDA published a release titled, “FDA puts company on notice for marketing unapproved stem cell products for treating serious conditions.”14 In it, the FDA Acting Commissioner Ned Sharpless, M.D., said:
We continue to see companies and individuals use questionable marketing campaigns to take advantage of vulnerable patients and their families with unproven claims about their unapproved stem cell products. The reality is that at this time, there isn’t enough evidence to support the use of stem cells for purposes other than reconstitution of blood formation and the immune system.15
Yet, the FDA has created an inconsistent regulatory environment in the stem cell arena. The warning it gave to R3 Stem Cell in response to press attention seems to have gone without follow-up.
In addition to the national press attention from The New Yorker and ProPublica report and from Pew, local news outlets have been reporting on the dangers of these therapies. The Phoenix New Times reported critically on Greene specifically in May 2019.16 Texas Monthly reported in January 2020 on how regenerative medicine has become a $2 billion industry and the people it has harmed—physically and economically.17
He has muddied the waters in terms of stem cell services: customers believe his marketing and that they could be helped by his products.
- 1Adrienne Biddings, “A New Policy on Advertising for Speculative and Experimental Medical Treatments,” Google Ads Help, September 6, 2019, archived on Perma.cc, http://perma.cc/SLB6-RZW5.
- 2R3 Stem Cell, Stem Cell Facebook Marketing Now That Google Banned Stem Cell Ads (YouTube, February 9, 2020), archived on Perma.cc, https://perma.cc/P8KW-7MG7.
- 3“Cord Blood Banking,” The American College of Obstetricians and Gynecologists, February 2021, archived on Perma.cc, https://perma.cc/AJ8L-PBH6.
- 4“Cord Blood Banking - Information for Consumers,” FDA, July 23, 2012, archived on Perma.cc, https://perma.cc/9HJ7-WG22.
- 5“Frequently Asked Questions,” Hawai’i Cord Blood Bank, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/9EWY-N6B2.
- 6“Donating Umbilical Cord Blood to a Public Bank,” Health Resources & Services Administration, November 1, 2019, archived on Perma.cc, https://perma.cc/NA3J-F28W.
- 7“Can I Donate Cord Blood?,” Be the Match, June 7, 2021, https://perma.cc/6Y6P-CGW7; “Cord Blood and Transplants,” Be the Match, August 9, 2021, archived on Perma.cc, https://perma.cc/7QHB-C63J.
- 8Denver7, “Stem Cells: Lots of Hype, but Do They Work?,” The Denver Channel, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/6YKZ-SNQV.
- 9Chen, “The Birth-Tissue Profiteers.”
- 10Chen.
- 11 Liz Richardson, “Limited Reporting of Adverse Events Tied to Regenerative Treatments Leaves Consumers Vulnerable,” Pew, July 31, 2020, archived on Perma.cc, https://perma.cc/Q49C-FRVV; Liz Richardson, “FDA’s Framework for Regulating Regenerative Medicine Will Improve Oversight,” Pew, October 17, 2019, archived on Perma.cc, https://perma.cc/H39A-SKA6.
- 12 Caroline Chen, “Citing ‘Safety Concerns,’ FDA Cautions National Marketer of Unproven Stem Cell Treatments,” ProPublica, May 31, 2019,archived on Perma.cc, https://perma.cc/VJ9V-XF9S.
- 13FDA, “Untitled Letter,” May 28, 2019, archived on Perma.cc, https://perma.cc/U43Q-PR6A.
- 14“FDA Puts Company on Notice for Marketing Unapproved Stem Cell Products for Treating Serious Conditions,” FDA, May 30, 2019, archived on Perma.cc, https://perma.cc/5ABW-AWL6.
- 15“FDA Puts Company on Notice for Marketing Unapproved Stem Cell Products for Treating Serious Conditions.”
- 16Meg O’Connor, “Deadly Arizona Doc Hawks Bogus Stem Cell Treatment,” Phoenix New Times, May 17, 2019, archived on Perma.cc, https://perma.cc/723V-Q97Q.
- 17Laura Bell, “How Unproven Stem Cell Therapies Are Costing Desperate Patients,” Texas Monthly, January 2020, archived on Perma.cc, https://perma.cc/MEX5-AFV8.
STAGE 5: Adjustments by campaign operators
After critical press and FDA condemnation in 2019, Greene redeployed his tactic of burying the news online. He purchased advertisements and press releases that were distributed via local news websites. He did so more aggressively than before critical press coverage of him: faux articles, or advertorials, dated after the ProPublica/New Yorker report far exceed the number of articles dated before it.
For example, a paid advertisement in Salisbury, Maryland’s WBOC is titled “The Modern Evolution of Regenerative Medicine Treatment with Dr. David Greene and R3 Stem Cell.”1 In small grey lettering, there is a notice that this is sponsored content. The advertorial praises Greene’s work and attempts to combat critical reporting on R3’s procedures. A line from the article reads, “It is also possible that you might have received wavering in your mind about this magical savior of mankind.”2 By acknowledging that there is critical reporting about him, he attempts to discredit it by calling it “disinformation.”
It can be hard to tell that these apparent “sponsored content” articles are in fact advertisements (the “sponsored content” notice is often smaller and subtler than the headline or article text), which may cause some readers to believe that their local news station is recommending R3. These paid advertisements are also tweeted by the press release service.
- 1No longer active: archived on Perma.cc, https://perma.cc/76PG-3FBS.
- 2No longer active; archived on Perma.cc, https://perma.cc/76PG-3FBS.
This happens over and over: in Reno, Nevada’s KTVN, there’s “R3 Stem Cell Wins 2021 Most Outstanding Regenerative Medicine Company Award,”1 “Dr. David Greene and R3 Stem Cell Builds Success Stories With Regenerative Medicine,”2 and “R3 Stem Cell and Dr. David Greene Open Regenerative Clinic in Cancun Mexico;”3 in Vestal, New York’s WICZ there’s “R3 Medical Training Announces MSK Ultrasound Injection Course Registration is Open for April 2021 Courses,”4 Yahoo! Finance ran one of his ads, announcing, “R3 International Offering New Stem Cell Therapy Program for Kidney Disease in Mexico,”5 and the AP published “R3 International Now Offering Stem Cell Therapy for Coronavirus in Mexico.”6 These advertorials, all posted online during early research on this case study in Spring 2021, no longer exist.
- 1 No longer active: archived on Perma.cc, https://perma.cc/LNK7-WKUN.
- 2 No longer active: archived on Perma.cc, https://perma.cc/6EQN-294M.
- 3 No longer active: archived on Perma.cc, https://perma.cc/78Q9-4WLR.
- 4 No longer active: archived on Perma.cc, https://perma.cc/S7A4-XTTU.
- 5 No longer active: archived on Perma.cc, https://perma.cc/VSC3-LL99.
- 6 R3 Stem Cell, “R3 International Now Offering Stem Cell Therapy for Coronavirus in Mexico,” AP NEWS, June 30, 2020, archived on Perma.cc, https://perma.cc/9E3M-ZDC2.
With this tactic, Greene has been able to push bad press about him lower into the search results. A Google or Yahoo! search for “R3 David Green” now returns his LinkedIn page, a few versions of his website, and a sponsored content that is meant to look like a genuine article.
Most recently, Greene’s R3 was awarded “Most Outstanding Regenerative Medicine Company Award for 2021” by Corporate Vision.1 Corporate Vision is also a pay-to-play award/vanity scam, according to 2 and to BlueSky, a PR and communications firm.3
A tactical adaptation has been to expand R3’s medical operations beyond the US healthcare system and FDA. Greene has opened clinics in Mexico, Pakistan, Canada, Egypt, and the Philippines, where patients can get “millions of umbilical cord derived stem cells and billions of exosomes for a fraction of U.S. cost!”4 While he promotes his international expansion as affordability-driven, it is part of a larger network of “stem cell tourism,” where clinics are established in countries that have little or no regulation on such injections.5
Unlike his Twitter account for his US-based clinics, Twitter has allowed its Pakistan-based account (@R3StemCellPK) to remain active. The account was created in March 2021. It has no followers and has posted 48 tweets.6 R3 Pakistan Facebook (3.8K followers) and YouTube accounts (74 subscribers) appear unmitigated. R3 Mexico links to the remaining US R3 accounts (Facebook and YouTube).
Lastly, although Greene continues to use the honorific “Dr.” despite losing his certification, he bought the title back. He enrolled in and graduated from Panama College of Stem Cell’s PhD program, per his LinkedIn. It is a three-year, virtual, unaccredited program that costs about $8,000.7 He is now advertising himself as “CEO David Greene, MD, PhD, MBA.”
Special thanks to Dr. Ralda Nehme for reviewing this case.
- 1“R3 Stem Cell Wins 2021 Most Outstanding Regenerative Medicine Company Award,” R3 Stem Cell, February 26, 2021, archived on Perma.cc, https://perma.cc/QY98-LQQJ.
- 2u/astronautvibes, “Business Award Looks like a ‘Vanity Scam’ / Not an Actual Award. Anyone Have Details on This? A Client of Mine Says They’ve Won but I’m Afraid Someone Is Taking Their Money.,” Reddit Post, R/Scams, June 24, 2020, archived on Perma.cc, https://perma.cc/6TZW-SDJ8.
- 3Fahida Begum, “Recruiters, Beware of Fake Awards,” BlueSky PR, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/8DP5-QVLD.
- 4“Find a Doctor,” R3 Stem Cell, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/9CZX-3USR.
- 5Gina Kolata, “A Cautionary Tale of ‘Stem Cell Tourism,’” New York Times, June 22, 2016, archived on Perma.cc, https://perma.cc/U5T7-W75E.
- 6“R3 Stem Cell Pakistan (@R3StemCellPK),” Twitter, accessed September 24, 2021, archived on Perma.cc, https://perma.cc/YRR2-YSYG.
- 7Michael Hiltzik, “Column: Backing the FDA, a Federal Judge Delivers a Blow against Bogus Stem Cell Clinics,” Los Angeles Times, June 3, 2019, archived on Perma.cc, https://perma.cc/G7HC-ASRK.
Cite this case study
Jennifer Nilsen, "Cord Blood and Medical Misinformation: The Big Business of Unproven Stem Cell Treatments," The Media Manipulation Case Book, November 17, 2021, https://casebook-static.pages.dev/case-studies/cord-blood-and-medical-misinformation-big-business-unproven-stem-cell-treatments.